Part:BBa_K729006:Experience
This experience page is provided so that any user may enter their experience using this part.
Please enter
how you used this part and how it worked out.
Applications of BBa_K729006
User Reviews
UNIQ829623020cadbfd5-partinfo-00000000-QINU UNIQ829623020cadbfd5-partinfo-00000001-QINU
|
•••••
iGEM Bielefeld |
Sequencing analysisFor the improvement of the data collection the BioBrick of the University College London iGEM team was sequenced. The sequence analysis shows that the analyzed BioBrick matches the sequence published in the Partsregistry (see Fig. 1). It was proven that the BioBrick codes for the E. coli laccase CueO, indeed. Therefore the sequence was aligned with CueO from E. coli BL21(DE3). It matched with 100 %.  Figure 1: A short part of the sequencing analysis results of the BioBrick BBa_K729006. Cultivation Figure 2: Comparison of the cultivated E. coli KRX and E. coli KRX containing BBa_K729006 cultivations. The OD600 was measured each hour for 12 h. E. coli KRX was transformed with the BioBrick BBa_K729006 of the UCL team. The cells were characterized by growth kinetics and activity test. E. coli KRX containing BBa_K729006 was cultivated in [http://2012.igem.org/Team:Bielefeld-Germany/Protocols/Materials#LB_medium LB-medium] with a total volume of 60 mL for 12 h at 37 °C (120 rpm) in 250 mL shaking flasks without baffles. To produce a higher amount of the protein we also cultivated a total volume of 200 mL in a 1 L shaking flask without baffles. The OD600 values were determined each hour. To measure the influence of the transformed BioBrick on the growth of the cells a negative control (E. coli KRX) was cultivated identically. In Fig. 2 the growth curves ofE. coli KRX containing BBa_K729006 and of the negative control E. coli KRX are plotted. As expected the E. coli KRX containing BBa_K729006 grew slower than the negative control. This can be explained by the protein expression. The cells were harvested and centrifuged after 12 h. The pellets were resuspended in 100 mM Na-Acetat-buffer, lysed by [http://2012.igem.org/Team:Bielefeld-Germany/Protocols/Production#Mechanical_lysis sonication] and centrifuged. The supernatant was analyzed by SDS-PAGE and laccase activity assay. SDS-PAGE Figure 3: SDS-PAGE of the lysates of E. coli KRX with BBa_K729006, E. coli KRX (negative control) and with BBa_K863005 (positive control). ECOL has a molecular weight of 53.4 kDa. In Fig. 3 the SDS-PAGE of the E. coli KRX lysates with BBa_K729006, BBa_K863005 and without any BioBrick (negative control) are shown after they have been treated the same way. No clear distinction between the samples is possible. By comparing the bands at 53.4 kDa (which matches the molecular weight of ECOL) just a small difference between the intensities of the bands can be considered. The E. coli KRX containing BBa_K863005 has a slightly stronger band. Laccase activity testsLaccase activity tests were performed by using 198 µL of the sample (supernatant of the lysed cells containing BBa_K729006 and BBa_K863005) and 2 µL ABTS (20 mM ABTS stock). Since laccases are capable of oxidizing ABTS the activity of laccases can be detected by measuring the absorption of oxidized ABTS at 420 nm. As a positive control the E. coli KRX strain containing BBa_K863005 which already has been [http://2012.igem.org/Team:Bielefeld-Germany/Results/Summary#4 characterized] by the team was applied as well. Using this approach there was no detectable activity over a period of 40 minutes (see Fig. 4). To gain activity the samples were incubated with 0.4 mM CuCl2 for at least 2 hours. After incubation time another setting was started using 198 µL of the samples containing BBa_K729006 and BBa_K863005 and applied 0.1 mM ABTS. The measurements lasted for 5 hours but the results did not show a decrease in oxidized ABTS again (see Fig. 5). A possible reason can be contaminants in the samples, e.g. salts or low molecular agents, that could have affected the activity of the tested laccases. The samples were rebuffered in deionized H2O using HiTrap Desalting Columns and then again incubated with 0.4 mM CuCl2 for 2 hours. This time the measurement setup differed because no buffer remained in the samples. The new measurements were performed with 100 mM sodium acetate buffer (pH 5), 158 µL sample and 0.01 mM ABTS for 5 hours at a temperature of 25°C. This sort of sample preparation led to oxidizing activity of the laccases and therefore to an increase in oxidized ABTS (see Fig. 6). After 5 hours both laccases BBa_K729006 and BBa_K863005 showed high activity and oxidized around 8 µM ABTS. Since both laccases derive from the same organism they both show a similar reaction behavior.  Figure 4: Activity measurement of BBa_K729006 and BBa_K863005 using ABTS over a time period of 40 minutes. No increase in oxidized ABTS was detectable. 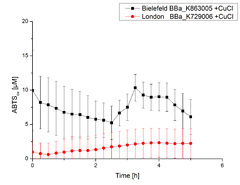 Figure 5: Activity measurement of BBa_K729006 and BBa_K863005 after 2 hours incubation with 0.4 mM CuCl2 using ABTS over a time period of 5 hours. No activity of both laccases could be detected since there was no increase in oxidized ABTS.  Figure 6: Activity measurement of BBa_K729006 and BBa_K863005 after desalting and 2 hours incubation with 0.4 mM CuCl2 using ABTS over a time period of 5 hours. A considerable increase in oxidized ABTS is noticeable. After 5 hours both laccases were capable of oxidizing 8 µM ABTS. |
